
Q < 1 in this case, so the process is spontaneous. The Nernst equation finds the cell potential of a reaction, or how much power a reaction will have at any given moment. Nernst Equation Calculator Nernst Equation at Room Temperature: E E 0. To get a positive cell potential (spontaneous process) the reaction quotient Q must be <1.

We will now extend electrochemistry by determining the relationship between =+0.021 VĪnd the process is spontaneous at these conditions.Ĭheck your answer: In a concentration cell, the standard cell potential will always be zero.
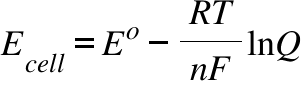
Relate cell potentials to free energy changes z valency of ion (Na + is plus one, Ca 2+ is plus two and Cl - is minus one) So the value 58 mV comes not out of thin air but from the terms in equation 3.
#NERNST EQUATION CALCULATOR FULL#
By the end of this module, you will be able to: In electrochemistry, the Nernst equation is an equation that relates the reduction potential of an electrochemical reaction (half-cell or full cell reaction) to.


 0 kommentar(er)
0 kommentar(er)
